Introduction
Randomized controlled study
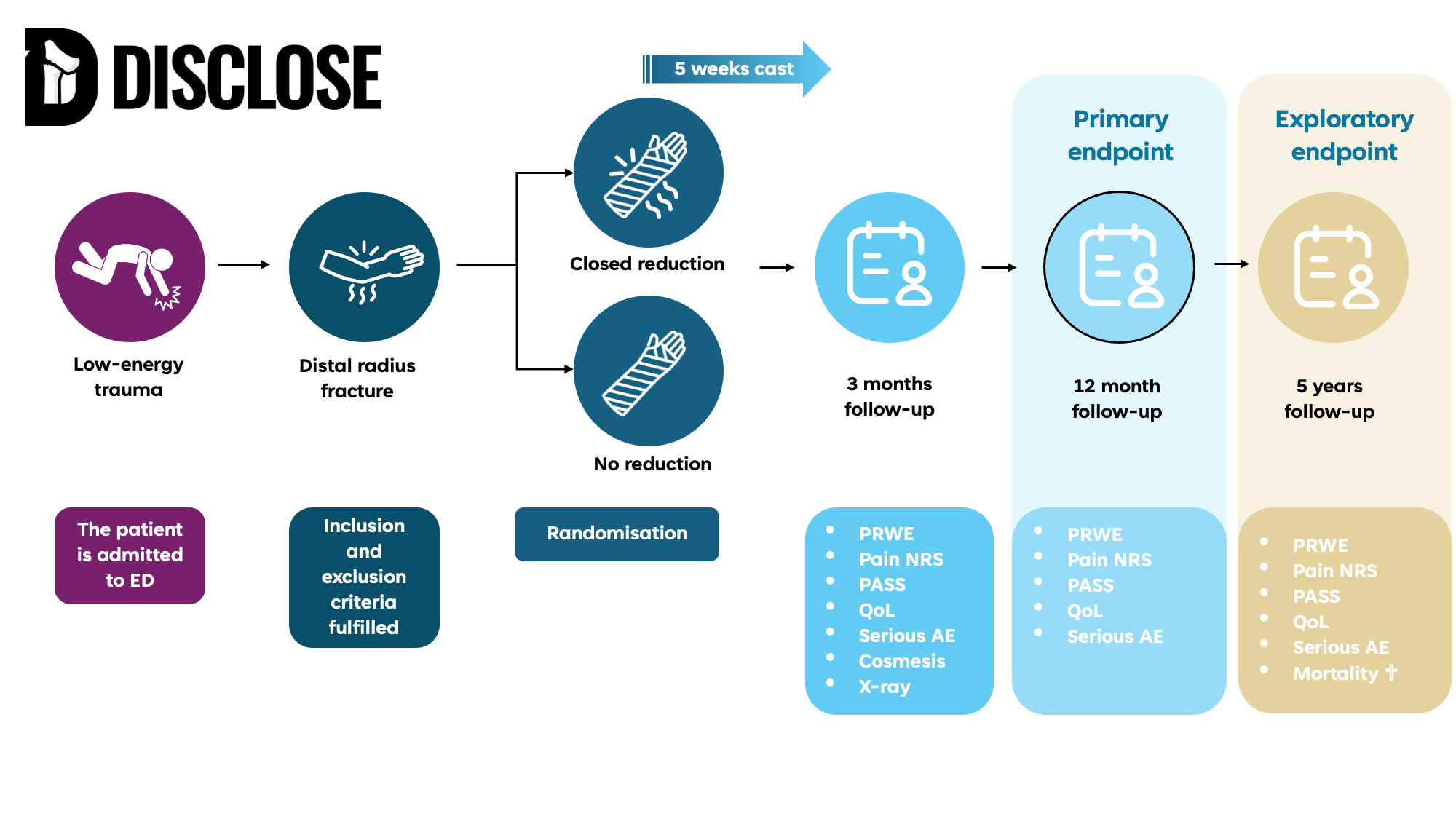
The DISCLOSE trial aims to evaluate whether casting without prior closed reduction is equivalent to casting following closed reduction with respect to wrist-related pain and disability, as measured by the Patient-Rated Wrist Evaluation (PRWE) score at 12 months, in patients aged 65 years or older with a displaced distal radius fracture.
Patients
We are recruiting patients aged 65 years or older who have sustained a low-energy, displaced distal radius fracture. Participants must be living independently and able to provide informed consent. Several exclusion criteria apply, including patients with other significant injuries, working individuals, or those with certain types of fractures.
Intervention and Control
Participants are randomly assigned to one of two groups
No reduction
A dorsal cast is applied without attempting to reposition the fracture. The cast is removed after 5 weeks, and patients are encouraged to begin hand movement and follow a standardized home exercise protocol.
Closed reduction
The fracture is reduced under local anaesthesia in the emergency department before applying the cast. As in the intervention group, the cast is removed after 5 weeks, followed by the same exercise protocol.
Patients who decline randomization are offered the option to join an observational cohort, where they receive standard care and are followed similarly to those in the trial arms.
Endpoints
Primary endpoint
The primary endpoint is wrist function at 12 months, assessed using the Patient-Rated Wrist Evaluation (PRWE) score.
Key secondary endpoints
- Pain - Numeric Rating Scale (NRS) at 3 months and 1 year
- Patient satisfaction (PASS) at 3 months and 1 year
- Quality of Life (EQ-5D-5L Index) at 3 months and 1 year
- Serious adverse events at 3 months and 1 year
- Cosmesis at 3 months
Other endpoints
- Radiographic measurements at 3 months
Hospitals
The study is coordinated by Tampere University Hospital in Finland and conducted across multiple Scandinavian centers in Finland, Denmark, Sweden, and Estonia. This international collaboration ensures robust data and a wide range of patient perspectives across Nordic healthcare systems.
- Locations
- 8
- Countries
- 4
- Researchers
- 15 +